Living Organisms R4equire a Continual Supply of Energy to Exist Becaue Quizlet
As we have just seen, cells require a constant supply of energy to generate and maintain the biological order that keeps them alive. This energy is derived from the chemical bond energy in food molecules, which thereby serve as fuel for cells.
Sugars are particularly important fuel molecules, and they are oxidized in small steps to carbon dioxide (CO2) and water (Figure 2-69). In this section we trace the major steps in the breakdown, or catabolism, of sugars and show how they produce ATP, NADH, and other activated carrier molecules in animal cells. We concentrate on glucose breakdown, since it dominates energy production in most animal cells. A very similar pathway also operates in plants, fungi, and many bacteria. Other molecules, such as fatty acids and proteins, can also serve as energy sources when they are funneled through appropriate enzymatic pathways.

Figure 2-69
Schematic representation of the controlled stepwise oxidation of sugar in a cell, compared with ordinary burning. (A) In the cell, enzymes catalyze oxidation via a series of small steps in which free energy is transferred in conveniently sized packets (more...)
Food Molecules Are Broken Down in Three Stages to Produce ATP
The proteins, lipids, and polysaccharides that make up most of the food we eat must be broken down into smaller molecules before our cells can use them—either as a source of energy or as building blocks for other molecules. The breakdown processes must act on food taken in from outside, but not on the macromolecules inside our own cells. Stage 1 in the enzymatic breakdown of food molecules is therefore digestion, which occurs either in our intestine outside cells, or in a specialized organelle within cells, the lysosome. (A membrane that surrounds the lysosome keeps its digestive enzymes separated from the cytosol, as described in Chapter 13.) In either case, the large polymeric molecules in food are broken down during digestion into their monomer subunits—proteins into amino acids, polysaccharides into sugars, and fats into fatty acids and glycerol—through the action of enzymes. After digestion, the small organic molecules derived from food enter the cytosol of the cell, where their gradual oxidation begins. As illustrated in Figure 2-70, oxidation occurs in two further stages of cellular catabolism: stage 2 starts in the cytosol and ends in the major energy-converting organelle, the mitochondrion; stage 3 is entirely confined to the mitochondrion.
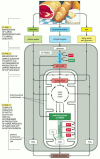
Figure 2-70
Simplified diagram of the three stages of cellular metabolism that lead from food to waste products in animal cells. This series of reactions produces ATP, which is then used to drive biosynthetic reactions and other energy-requiring processes in the (more...)
In stage 2 a chain of reactions called glycolysis converts each molecule of glucose into two smaller molecules of pyruvate. Sugars other than glucose are similarly converted to pyruvate after their conversion to one of the sugar intermediates in this glycolytic pathway. During pyruvate formation, two types of activated carrier molecules are produced—ATP and NADH. The pyruvate then passes from the cytosol into mitochondria. There, each pyruvate molecule is converted into CO2 plus a two-carbon acetyl group—which becomes attached to coenzyme A (CoA), forming acetyl CoA, another activated carrier molecule (see Figure 2-62). Large amounts of acetyl CoA are also produced by the stepwise breakdown and oxidation of fatty acids derived from fats, which are carried in the bloodstream, imported into cells as fatty acids, and then moved into mitochondria for acetyl CoA production.
Stage 3 of the oxidative breakdown of food molecules takes place entirely in mitochondria. The acetyl group in acetyl CoA is linked to coenzyme A through a high-energy linkage, and it is therefore easily transferable to other molecules. After its transfer to the four-carbon molecule oxaloacetate, the acetyl group enters a series of reactions called the citric acid cycle. As we discuss shortly, the acetyl group is oxidized to CO2 in these reactions, and large amounts of the electron carrier NADH are generated. Finally, the high-energy electrons from NADH are passed along an electron-transport chain within the mitochondrial inner membrane, where the energy released by their transfer is used to drive a process that produces ATP and consumes molecular oxygen (O2). It is in these final steps that most of the energy released by oxidation is harnessed to produce most of the cell's ATP.
Because the energy to drive ATP synthesis in mitochondria ultimately derives from the oxidative breakdown of food molecules, the phosphorylation of ADP to form ATP that is driven by electron transport in the mitochondrion is known as oxidative phosphorylation. The fascinating events that occur within the mitochondrial inner membrane during oxidative phosphorylation are the major focus of Chapter 14.
Through the production of ATP, the energy derived from the breakdown of sugars and fats is redistributed as packets of chemical energy in a form convenient for use elsewhere in the cell. Roughly 109 molecules of ATP are in solution in a typical cell at any instant, and in many cells, all this ATP is turned over (that is, used up and replaced) every 1–2 minutes.
In all, nearly half of the energy that could in theory be derived from the oxidation of glucose or fatty acids to H2O and CO2 is captured and used to drive the energetically unfavorable reaction Pi + ADP → ATP. (By contrast, a typical combustion engine, such as a car engine, can convert no more than 20% of the available energy in its fuel into useful work.) The rest of the energy is released by the cell as heat, making our bodies warm.
Glycolysis Is a Central ATP-producing Pathway
The most important process in stage 2 of the breakdown of food molecules is the degradation of glucose in the sequence of reactions known as glycolysis—from the Greek glukus, "sweet," and lusis, "rupture." Glycolysis produces ATP without the involvement of molecular oxygen (O2 gas). It occurs in the cytosol of most cells, including many anaerobic microorganisms (those that can live without utilizing molecular oxygen). Glycolysis probably evolved early in the history of life, before the activities of photosynthetic organisms introduced oxygen into the atmosphere. During glycolysis, a glucose molecule with six carbon atoms is converted into two molecules of pyruvate, each of which contains three carbon atoms. For each molecule of glucose, two molecules of ATP are hydrolyzed to provide energy to drive the early steps, but four molecules of ATP are produced in the later steps. At the end of glycolysis, there is consequently a net gain of two molecules of ATP for each glucose molecule broken down.
The glycolytic pathway is presented in outline in Figure 2-71, and in more detail in Panel 2-8 (pp. 124–125). Glycolysis involves a sequence of 10 separate reactions, each producing a different sugar intermediate and each catalyzed by a different enzyme. Like most enzymes, these enzymes all have names ending in ase—like isomerase and dehydrogenase—which indicate the type of reaction they catalyze.
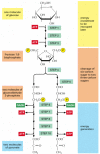
Figure 2-71
An outline of glycolysis. Each of the 10 steps shown is catalyzed by a different enzyme. Note that step 4 cleaves a six-carbon sugar into two three-carbon sugars, so that the number of molecules at every stage after this doubles. As indicated, step 6 (more...)
![]()
Panel 2-8
Details of the 10 Steps of Glycolysis.
Although no molecular oxygen is involved in glycolysis, oxidation occurs, in that electrons are removed by NAD+ (producing NADH) from some of the carbons derived from the glucose molecule. The stepwise nature of the process allows the energy of oxidation to be released in small packets, so that much of it can be stored in activated carrier molecules rather than all of it being released as heat (see Figure 2-69). Thus, some of the energy released by oxidation drives the direct synthesis of ATP molecules from ADP and Pi, and some remains with the electrons in the high-energy electron carrier NADH.
Two molecules of NADH are formed per molecule of glucose in the course of glycolysis. In aerobic organisms (those that require molecular oxygen to live), these NADH molecules donate their electrons to the electron-transport chain described in Chapter 14, and the NAD+ formed from the NADH is used again for glycolysis (see step 6 in Panel 2-8, pp. 124–125).
Fermentations Allow ATP to Be Produced in the Absence of Oxygen
For most animal and plant cells, glycolysis is only a prelude to the third and final stage of the breakdown of food molecules. In these cells, the pyruvate formed at the last step of stage 2 is rapidly transported into the mitochondria, where it is converted into CO2 plus acetyl CoA, which is then completely oxidized to CO2 and H2O.
In contrast, for many anaerobic organisms—which do not utilize molecular oxygen and can grow and divide without it—glycolysis is the principal source of the cell's ATP. This is also true for certain animal tissues, such as skeletal muscle, that can continue to function when molecular oxygen is limiting. In these anaerobic conditions, the pyruvate and the NADH electrons stay in the cytosol. The pyruvate is converted into products excreted from the cell—for example, into ethanol and CO2 in the yeasts used in brewing and breadmaking, or into lactate in muscle. In this process, the NADH gives up its electrons and is converted back into NAD+. This regeneration of NAD+ is required to maintain the reactions of glycolysis (Figure 2-72).
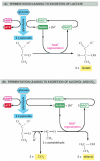
Figure 2-72
Two pathways for the anaerobic breakdown of pyruvate. (A) When inadequate oxygen is present, for example, in a muscle cell undergoing vigorous contraction, the pyruvate produced by glycolysis is converted to lactate as shown. This reaction regenerates (more...)
Anaerobic energy-yielding pathways like these are called fermentations. Studies of the commercially important fermentations carried out by yeasts inspired much of early biochemistry. Work in the nineteenth century led in 1896 to the then startling recognition that these processes could be studied outside living organisms, in cell extracts. This revolutionary discovery eventually made it possible to dissect out and study each of the individual reactions in the fermentation process. The piecing together of the complete glycolytic pathway in the 1930s was a major triumph of biochemistry, and it was quickly followed by the recognition of the central role of ATP in cellular processes. Thus, most of the fundamental concepts discussed in this chapter have been understood for more than 50 years.
Glycolysis Illustrates How Enzymes Couple Oxidation to Energy Storage
We have previously used a "paddle wheel" analogy to explain how cells harvest useful energy from the oxidation of organic molecules by using enzymes to couple an energetically unfavorable reaction to an energetically favorable one (see Figure 2-56). Enzymes play the part of the paddle wheel in our analogy, and we now return to a step in glycolysis that we have previously discussed, in order to illustrate exactly how coupled reactions occur.
Two central reactions in glycolysis (steps 6 and 7) convert the three-carbon sugar intermediate glyceraldehyde 3-phosphate (an aldehyde) into 3-phosphoglycerate (a carboxylic acid). This entails the oxidation of an aldehyde group to a carboxylic acid group, which occurs in two steps. The overall reaction releases enough free energy to convert a molecule of ADP to ATP and to transfer two electrons from the aldehyde to NAD+ to form NADH, while still releasing enough heat to the environment to make the overall reaction energetically favorable (ΔG° for the overall reaction is -3.0 kcal/mole).
The pathway by which this remarkable feat is accomplished is outlined in Figure 2-73. The chemical reactions are guided by two enzymes to which the sugar intermediates are tightly bound. The first enzyme (glyceraldehyde 3-phosphate dehydrogenase) forms a short-lived covalent bond to the aldehyde through a reactive -SH group on the enzyme, and it catalyzes the oxidation of this aldehyde while still in the attached state. The high-energy enzyme-substrate bond created by the oxidation is then displaced by an inorganic phosphate ion to produce a high-energy sugar-phosphate intermediate, which is thereby released from the enzyme. This intermediate then binds to the second enzyme (phosphoglycerate kinase). This enzyme catalyzes the energetically favorable transfer of the high-energy phosphate just created to ADP, forming ATP and completing the process of oxidizing an aldehyde to a carboxylic acid (see Figure 2-73).

Figure 2-73
Energy storage in steps 6 and 7 of glycolysis. In these steps the oxidation of an aldehyde to a carboxylic acid is coupled to the formation of ATP and NADH. (A) Step 6 begins with the formation of a covalent bond between the substrate (glyceraldehyde (more...)
We have shown this particular oxidation process in some detail because it provides a clear example of enzyme-mediated energy storage through coupled reactions (Figure 2-74). These reactions (steps 6 and 7) are the only ones in glycolysis that create a high-energy phosphate linkage directly from inorganic phosphate. As such, they account for the net yield of two ATP molecules and two NADH molecules per molecule of glucose (see Panel 2-8, pp. 124–125).

Figure 2-74
Schematic view of the coupled reactions that form NADH and ATP in steps 6 and 7 of glycolysis. The C-H bond oxidation energy drives the formation of both NADH and a high-energy phosphate bond. The breakage of the high-energy bond then drives ATP formation. (more...)
As we have just seen, ATP can be formed readily from ADP when reaction intermediates are formed with higher-energy phosphate bonds than those in ATP. Phosphate bonds can be ordered in energy by comparing the standard free-energy change (ΔG°) for the breakage of each bond by hydrolysis. Figure 2-75 compares the high-energy phosphoanhydride bonds in ATP with other phosphate bonds, several of which are generated during glycolysis.

Figure 2-75
Some phosphate bond energies. The transfer of a phosphate group from any molecule 1 to any molecule 2 is energetically favorable if the standard free-energy change (ΔG°) for the hydrolysis of the phosphate bond in molecule 1 is more negative (more...)
Sugars and Fats Are Both Degraded to Acetyl CoA in Mitochondria
We now move on to consider stage 3 of catabolism, a process that requires abundant molecular oxygen (O2 gas). Since the Earth is thought to have developed an atmosphere containing O2 gas between one and two billion years ago, whereas abundant life-forms are known to have existed on the Earth for 3.5 billion years, the use of O2 in the reactions that we discuss next is thought to be of relatively recent origin. In contrast, the mechanism used to produce ATP in Figure 2-73 does not require oxygen, and relatives of this elegant pair of coupled reactions could have arisen very early in the history of life on Earth.
In aerobic metabolism, the pyruvate produced by glycolysis is rapidly decarboxylated by a giant complex of three enzymes, called the pyruvate dehydrogenase complex. The products of pyruvate decarboxylation are a molecule of CO2 (a waste product), a molecule of NADH, and acetyl CoA. The three-enzyme complex is located in the mitochondria of eucaryotic cells; its structure and mode of action are outlined in Figure 2-76.

Figure 2-76
The oxidation of pyruvate to acetyl CoA and CO2. (A) The structure of the pyruvate dehydrogenase complex, which contains 60 polypeptide chains. This is an example of a large multienzyme complex in which reaction intermediates are passed directly from (more...)
The enzymes that degrade the fatty acids derived from fats likewise produce acetyl CoA in mitochondria. Each molecule of fatty acid (as the activated molecule fatty acyl CoA) is broken down completely by a cycle of reactions that trims two carbons at a time from its carboxyl end, generating one molecule of acetyl CoA for each turn of the cycle. A molecule of NADH and a molecule of FADH2 are also produced in this process (Figure 2-77).

Figure 2-77
The oxidation of fatty acids to acetyl CoA. (A) Electron micrograph of a lipid droplet in the cytoplasm (top), and the structure of fats (bottom). Fats are triacylglycerols. The glycerol portion, to which three fatty acids are linked through ester bonds, (more...)
Sugars and fats provide the major energy sources for most non-photosynthetic organisms, including humans. However, the majority of the useful energy that can be extracted from the oxidation of both types of foodstuffs remains stored in the acetyl CoA molecules that are produced by the two types of reactions just described. The citric acid cycle of reactions, in which the acetyl group in acetyl CoA is oxidized to CO2 and H2O, is therefore central to the energy metabolism of aerobic organisms. In eucaryotes these reactions all take place in mitochondria, the organelle to which pyruvate and fatty acids are directed for acetyl CoA production (Figure 2-78). We should therefore not be surprised to discover that the mitochondrion is the place where most of the ATP is produced in animal cells. In contrast, aerobic bacteria carry out all of their reactions in a single compartment, the cytosol, and it is here that the citric acid cycle takes place in these cells.

Figure 2-78
Pathways for the production of acetyl CoA from sugars and fats. The mitochondrion in eucaryotic cells is the place where acetyl CoA is produced from both types of major food molecules. It is therefore the place where most of the cell's oxidation reactions (more...)
The Citric Acid Cycle Generates NADH by Oxidizing Acetyl Groups to CO2
In the nineteenth century, biologists noticed that in the absence of air (anaerobic conditions) cells produce lactic acid (for example, in muscle) or ethanol (for example, in yeast), while in its presence (aerobic conditions) they consume O2 and produce CO2 and H2O. Intensive efforts to define the pathways of aerobic metabolism eventually focused on the oxidation of pyruvate and led in 1937 to the discovery of the citric acid cycle, also known as the tricarboxylic acid cycle or the Krebs cycle. The citric acid cycle accounts for about two-thirds of the total oxidation of carbon compounds in most cells, and its major end products are CO2 and high-energy electrons in the form of NADH. The CO2 is released as a waste product, while the high-energy electrons from NADH are passed to a membrane-bound electron-transport chain, eventually combining with O2 to produce H2O. Although the citric acid cycle itself does not use O2, it requires O2 in order to proceed because there is no other efficient way for the NADH to get rid of its electrons and thus regenerate the NAD+ that is needed to keep the cycle going.
The citric acid cycle, which takes place inside mitochondria in eucaryotic cells, results in the complete oxidation of the carbon atoms of the acetyl groups in acetyl CoA, converting them into CO2. But the acetyl group is not oxidized directly. Instead, this group is transferred from acetyl CoA to a larger, four-carbon molecule, oxaloacetate, to form the six-carbon tricarboxylic acid, citric acid, for which the subsequent cycle of reactions is named. The citric acid molecule is then gradually oxidized, allowing the energy of this oxidation to be harnessed to produce energy-rich activated carrier molecules. The chain of eight reactions forms a cycle because at the end the oxaloacetate is regenerated and enters a new turn of the cycle, as shown in outline in Figure 2-79.

Figure 2-79
Simple overview of the citric acid cycle. The reaction of acetyl CoA with oxaloacetate starts the cycle by producing citrate (citric acid). In each turn of the cycle, two molecules of CO2 are produced as waste products, plus three molecules of NADH, one (more...)
We have thus far discussed only one of the three types of activated carrier molecules that are produced by the citric acid cycle, the NAD+-NADH pair (see Figure 2-60). In addition to three molecules of NADH, each turn of the cycle also produces one molecule of FADH 2 (reduced flavin adenine dinucleotide) from FAD and one molecule of the ribonucleotide GTP (guanosine triphosphate) from GDP. The structures of these two activated carrier molecules are illustrated in Figure 2-80. GTP is a close relative of ATP, and the transfer of its terminal phosphate group to ADP produces one ATP molecule in each cycle. Like NADH, FADH2 is a carrier of high-energy electrons and hydrogen. As we discuss shortly, the energy that is stored in the readily transferred high-energy electrons of NADH and FADH2 will be utilized subsequently for ATP production through the process of oxidative phosphorylation, the only step in the oxidative catabolism of foodstuffs that directly requires gaseous oxygen (O2) from the atmosphere.
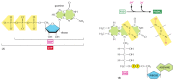
Figure 2-80
The structures of GTP and FADH2. (A) GTP and GDP are close relatives of ATP and ADP, respectively. (B) FADH2 is a carrier of hydrogens and high-energy electrons, like NADH and NADPH. It is shown here in its oxidized form (FAD) with the hydrogen-carrying (more...)
The complete citric acid cycle is presented in Panel 2-9 (pp. 126–127). The extra oxygen atoms required to make CO2 from the acetyl groups entering the citric acid cycle are supplied not by molecular oxygen, but by water. As illustrated in the panel, three molecules of water are split in each cycle, and the oxygen atoms of some of them are ultimately used to make CO2.
![]()
In addition to pyruvate and fatty acids, some amino acids pass from the cytosol into mitochondria, where they are also converted into acetyl CoA or one of the other intermediates of the citric acid cycle. Thus, in the eucaryotic cell, the mitochondrion is the center toward which all energy-yielding processes lead, whether they begin with sugars, fats, or proteins.
The citric acid cycle also functions as a starting point for important biosynthetic reactions by producing vital carbon-containing intermediates, such as oxaloacetate and α-ketoglutarate. Some of these substances produced by catabolism are transferred back from the mitochondrion to the cytosol, where they serve in anabolic reactions as precursors for the synthesis of many essential molecules, such as amino acids.
Electron Transport Drives the Synthesis of the Majority of the ATP in Most Cells
It is in the last step in the degradation of a food molecule that the major portion of its chemical energy is released. In this final process the electron carriers NADH and FADH2 transfer the electrons that they have gained when oxidizing other molecules to the electron-transport chain, which is embedded in the inner membrane of the mitochondrion. As the electrons pass along this long chain of specialized electron acceptor and donor molecules, they fall to successively lower energy states. The energy that the electrons release in this process is used to pump H+ ions (protons) across the membrane—from the inner mitochondrial compartment to the outside (Figure 2-81). A gradient of H+ ions is thereby generated. This gradient serves as a source of energy, being tapped like a battery to drive a variety of energy-requiring reactions. The most prominent of these reactions is the generation of ATP by the phosphorylation of ADP.

Figure 2-81
The generation of an H+ gradient across a membrane by electron-transport reactions. A high-energy electron (derived, for example, from the oxidation of a metabolite) is passed sequentially by carriers A, B, and C to a lower energy state. In this diagram (more...)
At the end of this series of electron transfers, the electrons are passed to molecules of oxygen gas (O2) that have diffused into the mitochondrion, which simultaneously combine with protons (H+) from the surrounding solution to produce molecules of water. The electrons have now reached their lowest energy level, and therefore all the available energy has been extracted from the food molecule being oxidized. This process, termed oxidative phosphorylation (Figure 2-82), also occurs in the plasma membrane of bacteria. As one of the most remarkable achievements of cellular evolution, it will be a central topic of Chapter 14.
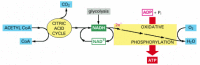
Figure 2-82
The final stages of oxidation of food molecules. Molecules of NADH and FADH2 (FADH2 is not shown) are produced by the citric acid cycle. These activated carriers donate high-energy electrons that are eventually used to reduce oxygen gas to water. A major (more...)
In total, the complete oxidation of a molecule of glucose to H2O and CO2 is used by the cell to produce about 30 molecules of ATP. In contrast, only 2 molecules of ATP are produced per molecule of glucose by glycolysis alone.
Organisms Store Food Molecules in Special Reservoirs
All organisms need to maintain a high ATP/ADP ratio, if biological order is to be maintained in their cells. Yet animals have only periodic access to food, and plants need to survive overnight without sunlight, without the possibility of sugar production from photosynthesis. For this reason, both plants and animals convert sugars and fats to special forms for storage (Figure 2-83).

Figure 2-83
The storage of sugars and fats in animal and plant cells. (A) The structures of starch and glycogen, the storage form of sugars in plants and animals, respectively. Both are storage polymers of the sugar glucose and differ only in the frequency of branch (more...)
To compensate for long periods of fasting, animals store fatty acids as fat droplets composed of water-insoluble triacylglycerols, largely in specialized fat cells. And for shorter-term storage, sugar is stored as glucose subunits in the large branched polysaccharide glycogen, which is present as small granules in the cytoplasm of many cells, including liver and muscle. The synthesis and degradation of glycogen are rapidly regulated according to need. When more ATP is needed than can be generated from the food molecules taken in from the bloodstream, cells break down glycogen in a reaction that produces glucose 1-phosphate, which enters glycolysis.
Quantitatively, fat is a far more important storage form than glycogen, in part because the oxidation of a gram of fat releases about twice as much energy as the oxidation of a gram of glycogen. Moreover, glycogen differs from fat in binding a great deal of water, producing a sixfold difference in the actual mass of glycogen required to store the same amount of energy as fat. An average adult human stores enough glycogen for only about a day of normal activities but enough fat to last for nearly a month. If our main fuel reservoir had to be carried as glycogen instead of fat, body weight would need to be increased by an average of about 60 pounds.
Most of our fat is stored in adipose tissue, from which it is released into the bloodstream for other cells to utilize as needed. The need arises after a period of not eating; even a normal overnight fast results in the mobilization of fat, so that in the morning most of the acetyl CoA entering the citric acid cycle is derived from fatty acids rather than from glucose. After a meal, however, most of the acetyl CoA entering the citric acid cycle comes from glucose derived from food, and any excess glucose is used to replenish depleted glycogen stores or to synthesize fats. (While animal cells readily convert sugars to fats, they cannot convert fatty acids to sugars.)
Although plants produce NADPH and ATP by photosynthesis, this important process occurs in a specialized organelle, called a chloroplast, which is isolated from the rest of the plant cell by a membrane that is impermeable to both types of activated carrier molecules. Moreover, the plant contains many other cells—such as those in the roots—that lack chloroplasts and therefore cannot produce their own sugars or ATP. Therefore, for most of its ATP production, the plant relies on an export of sugars from its chloroplasts to the mitochondria that are located in all cells of the plant. Most of the ATP needed by the plant is synthesized in these mitochondria and exported from them to the rest of the plant cell, using exactly the same pathways for the oxidative breakdown of sugars that are utilized by nonphotosynthetic organisms (Figure 2-84).

Figure 2-84
How the ATP needed for most plant cell metabolism is made. In plants, the chloroplasts and mitochondria collaborate to supply cells with metabolites and ATP.
During periods of excess photosynthetic capacity during the day, chloroplasts convert some of the sugars that they make into fats and into starch, a polymer of glucose analogous to the glycogen of animals. The fats in plants are triacylglycerols, just like the fats in animals, and differ only in the types of fatty acids that predominate. Fat and starch are both stored in the chloroplast as reservoirs to be mobilized as an energy source during periods of darkness (see Figure 2-83B).
The embryos inside plant seeds must live on stored sources of energy for a prolonged period, until they germinate to produce leaves that can harvest the energy in sunlight. For this reason plant seeds often contain especially large amounts of fats and starch—which makes them a major food source for animals, including ourselves (Figure 2-85).

Figure 2-85
Some plant seeds that serve as important foods for humans. Corn, nuts, and peas all contain rich stores of starch and fat that provide the young plant embryo in the seed with energy and building blocks for biosynthesis. (Courtesy of the John Innes Foundation.) (more...)
Amino Acids and Nucleotides Are Part of the Nitrogen Cycle
In our discussion so far we have concentrated mainly on carbohydrate metabolism. We have not yet considered the metabolism of nitrogen or sulfur. These two elements are constituents of proteins and nucleic acids, which are the two most important classes of macromolecules in the cell and make up approximately two-thirds of its dry weight. Atoms of nitrogen and sulfur pass from compound to compound and between organisms and their environment in a series of reversible cycles.
Although molecular nitrogen is abundant in the Earth's atmosphere, nitrogen is chemically unreactive as a gas. Only a few living species are able to incorporate it into organic molecules, a process called nitrogen fixation. Nitrogen fixation occurs in certain microorganisms and by some geophysical processes, such as lightning discharge. It is essential to the biosphere as a whole, for without it life would not exist on this planet. Only a small fraction of the nitrogenous compounds in today's organisms, however, is due to fresh products of nitrogen fixation from the atmosphere. Most organic nitrogen has been in circulation for some time, passing from one living organism to another. Thus present-day nitrogen-fixing reactions can be said to perform a "topping-up" function for the total nitrogen supply.
Vertebrates receive virtually all of their nitrogen in their dietary intake of proteins and nucleic acids. In the body these macromolecules are broken down to amino acids and the components of nucleotides, and the nitrogen they contain is used to produce new proteins and nucleic acids or utilized to make other molecules. About half of the 20 amino acids found in proteins are essential amino acids for vertebrates (Figure 2-86), which means that they cannot be synthesized from other ingredients of the diet. The others can be so synthesized, using a variety of raw materials, including intermediates of the citric acid cycle as described below. The essential amino acids are made by nonvertebrate organisms, usually by long and energetically expensive pathways that have been lost in the course of vertebrate evolution.

Figure 2-86
The nine essential amino acids. These cannot be synthesized by human cells and so must be supplied in the diet.
The nucleotides needed to make RNA and DNA can be synthesized using specialized biosynthetic pathways: there are no "essential nucleotides" that must be provided in the diet. All of the nitrogens in the purine and pyrimidine bases (as well as some of the carbons) are derived from the plentiful amino acids glutamine, aspartic acid, and glycine, whereas the ribose and deoxyribose sugars are derived from glucose.
Amino acids that are not utilized in biosynthesis can be oxidized to generate metabolic energy. Most of their carbon and hydrogen atoms eventually form CO2 or H2O, whereas their nitrogen atoms are shuttled through various forms and eventually appear as urea, which is excreted. Each amino acid is processed differently, and a whole constellation of enzymatic reactions exists for their catabolism.
Many Biosynthetic Pathways Begin with Glycolysis or the Citric Acid Cycle
Catabolism produces both energy for the cell and the building blocks from which many other molecules of the cell are made (see Figure 2-36). Thus far, our discussions of glycolysis and the citric acid cycle have emphasized energy production, rather than the provision of the starting materials for biosynthesis. But many of the intermediates formed in these reaction pathways are also siphoned off by other enzymes that use them to produce the amino acids, nucleotides, lipids, and other small organic molecules that the cell needs. Some idea of the complexity of this process can be gathered from Figure 2-87, which illustrates some of the branches from the central catabolic reactions that lead to biosyntheses.
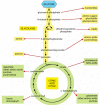
Figure 2-87
Glycolysis and the citric acid cycle provide the precursors needed to synthesize many important biological molecules. The amino acids, nucleotides, lipids, sugars, and other molecules—shown here as products—in turn serve as the precursors (more...)
The existence of so many branching pathways in the cell requires that the choices at each branch be carefully regulated, as we discuss next.
Metabolism Is Organized and Regulated
One gets a sense of the intricacy of a cell as a chemical machine from the relation of glycolysis and the citric acid cycle to the other metabolic pathways sketched out in Figure 2-88. This type of chart, which was used earlier in this chapter to introduce metabolism, represents only some of the enzymatic pathways in a cell. It is obvious that our discussion of cell metabolism has dealt with only a tiny fraction of cellular chemistry.

Figure 2-88
Glycolysis and the citric acid cycle are at the center of metabolism. Some 500 metabolic reactions of a typical cell are shown schematically with the reactions of glycolysis and the citric acid cycle in red. Other reactions either lead into these two (more...)
All these reactions occur in a cell that is less than 0.1 mm in diameter, and each requires a different enzyme. As is clear from Figure 2-88, the same molecule can often be part of many different pathways. Pyruvate, for example, is a substrate for half a dozen or more different enzymes, each of which modifies it chemically in a different way. One enzyme converts pyruvate to acetyl CoA, another to oxaloacetate; a third enzyme changes pyruvate to the amino acid alanine, a fourth to lactate, and so on. All of these different pathways compete for the same pyruvate molecule, and similar competitions for thousands of other small molecules go on at the same time. A better sense of this complexity can perhaps be attained from a three-dimensional metabolic map that allows the connections between pathways to be made more directly (Figure 2-89).
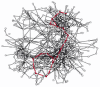
Figure 2-89
A representation of all of the known metabolic reactions involving small molecules in a yeast cell. As in Figure 2-88, the reactions of glycolysis and the citric acid cycle are highlighted in red. This metabolic map is unusual in making use of three-dimensions, (more...)
The situation is further complicated in a multicellular organism. Different cell types will in general require somewhat different sets of enzymes. And different tissues make distinct contributions to the chemistry of the organism as a whole. In addition to differences in specialized products such as hormones or antibodies, there are significant differences in the "common" metabolic pathways among various types of cells in the same organism.
Although virtually all cells contain the enzymes of glycolysis, the citric acid cycle, lipid synthesis and breakdown, and amino acid metabolism, the levels of these processes required in different tissues are not the same. For example, nerve cells, which are probably the most fastidious cells in the body, maintain almost no reserves of glycogen or fatty acids and rely almost entirely on a constant supply of glucose from the bloodstream. In contrast, liver cells supply glucose to actively contracting muscle cells and recycle the lactic acid produced by muscle cells back into glucose (Figure 2-90). All types of cells have their distinctive metabolic traits, and they cooperate extensively in the normal state, as well as in response to stress and starvation. One might think that the whole system would need to be so finely balanced that any minor upset, such as a temporary change in dietary intake, would be disastrous.
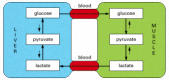
Figure 2-90
Schematic view of the metabolic cooperation between liver and muscle cells. The principal fuel of actively contracting muscle cells is glucose, much of which is supplied by liver cells. Lactic acid, the end product of anaerobic glucose breakdown by glycolysis (more...)
In fact, the metabolic balance of a cell is amazingly stable. Whenever the balance is perturbed, the cell reacts so as to restore the initial state. The cell can adapt and continue to function during starvation or disease. Mutations of many kinds can damage or even eliminate particular reaction pathways, and yet—provided that certain minimum requirements are met—the cell survives. It does so because an elaborate network of control mechanisms regulates and coordinates the rates of all of its reactions. These controls rest, ultimately, on the remarkable abilities of proteins to change their shape and their chemistry in response to changes in their immediate environment. The principles that underlie how large molecules such as proteins are built and the chemistry behind their regulation will be our next concern.
Summary
Glucose and other food molecules are broken down by controlled stepwise oxidation to provide chemical energy in the form of ATP and NADH. These are three main sets of reactions that act in series—the products of each being the starting material for the next: glycolysis (which occurs in the cytosol), the citric acid cycle (in the mitochondrial matrix), and oxidative phosphorylation (on the inner mitochondrial membrane). The intermediate products of glycolysis and the citric acid cycle are used both as sources of metabolic energy and to produce many of the small molecules used as the raw materials for biosynthesis. Cells store sugar molecules as glycogen in animals and starch in plants; both plants and animals also use fats extensively as a food store. These storage materials in turn serve as a major source of food for humans, along with the proteins that comprise the majority of the dry mass of the cells we eat.
Source: https://www.ncbi.nlm.nih.gov/books/NBK26882/
0 Response to "Living Organisms R4equire a Continual Supply of Energy to Exist Becaue Quizlet"
Post a Comment